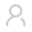标准化疗后总生存期25.6个月,而米哚妥林治疗后总生存期达到74.7个月,生存期延长2倍!
《壹篇》按


《新英格兰医学杂志》2017年6月23日在线先发
http://www.nejm.org/doi/full/10.1056/NEJMoa1614359
米哚妥林联合化疗治疗FLT3突变的急性髓性白血病背景
FLT3突变的急性髓性白血病患者预后差,米哚妥林(Midostaurin)是一种口服多激酶抑制剂,对FLT3突变患者有活性,我们进行了一项3期临床试验,研究标准化疗方案中加入米哚妥林是否可以延长这类患者的总生存期。
方法
我们对3277名18-59岁、新诊断出的急性髓性白血病患者进行了FLT3突变的筛查,将患者随机分组为标准化疗(用柔红霉素、阿糖胞苷进行诱导化疗,大剂量阿糖胞苷巩固治疗)+米哚妥林组或标准化疗+安慰剂组,巩固治疗后缓解的患者进入维持期,维持期期间患者接受米哚妥林或安慰剂。根据FLT3突变亚型、酪氨酸激酶结构域(TKD)点突变、高比值(>0.7)或低比值(0.05-0.7)野生型等位基因内部串联重复(ITD)突变(分别为[高]ITD、低[ITD])进行随机化分层。可以进行同种异体移植。主要终点为总生存期。
结果
共计717名患者进行了随机化分组,360名分入米哚妥林组、357名安慰剂组。FLT3亚型为214名患者(高)ITD、341名(低)ITD、162名TKD。就年龄、种族、FLT3亚型、细胞遗传学风险、血细胞计数而言,两个治疗组相当,但两组性别不同(米哚妥林组女性占51.7%、安慰剂组59.4%,P=0.04)。米哚妥林组的总生存期明显长于安慰剂组(死亡的比值比,0.78,单尾P=0.009),无事件生存期(自随机分组至复发、全因死亡、或获得完全缓解前的时间——译者查阅全文注)亦如此(进展或死亡的风险比,0.78,单尾P=0.002)。在两次主要分析和一次对进行了移植患者的数据进行审查的分析中发现,米哚妥林对所有FLT3亚型均获益。两组严重不良反应率相近。
结论
在FLT3突变的急性髓性白血病患者,标准化疗方案中加入多激酶抑制剂米哚妥林明显延长了总生存期和无病生存期。(由美国国家癌症研究所和诺华制药资助,ClinicalTrials.gov网站注册号NCT00651261。)
《壹篇》南南和北北


Richard M. Stone, M.D., Sumithra J. Mandrekar, Ph.D., Ben L. Sanford, M.S., Kristina Laumann, B.A., Susan Geyer, Ph.D., Clara D. Bloomfield, M.D., Christian Thiede, M.D., Thomas W. Prior, Ph.D., Konstanze Döhner, M.D., Guido Marcucci, M.D., Francesco Lo-Coco, M.D., Rebecca B. Klisovic, M.D., Andrew Wei, M.B., B.S., Ph.D., Jorge Sierra, M.D., Ph.D., Miguel A. Sanz, M.D., Ph.D., Joseph M. Brandwein, M.D., Theo de Witte, M.D., Dietger Niederwieser, M.D., Frederick R. Appelbaum, M.D., Bruno C. Medeiros, M.D., Martin S. Tallman, M.D., Jürgen Krauter, M.D., Richard F. Schlenk, M.D., Arnold Ganser, M.D., Hubert Serve, M.D., Gerhard Ehninger, M.D., Sergio Amadori, M.D., Richard A. Larson, M.D., and Hartmut Döhner, M.D.
June 23, 2017DOI: 10.1056/NEJMoa1614359
Background
Patients with acute myeloid leukemia (AML) and a FLT3 mutation have poor outcomes. We conducted a phase 3 trial to determine whether the addition of midostaurin — an oral multitargeted kinase inhibitor that is active in patients with a FLT3 mutation — to standard chemotherapy would prolong overall survival in this population.
Methods
We screened 3277 patients, 18 to 59 years of age, who had newly diagnosed AML for FLT3 mutations. Patients were randomly assigned to receive standard chemotherapy (induction therapy with daunorubicin and cytarabine and consolidation therapy with high-dose cytarabine) plus either midostaurin or placebo; those who were in remission after consolidation therapy entered a maintenance phase in which they received either midostaurin or placebo. Randomization was stratified according to subtype of FLT3 mutation: point mutation in the tyrosine kinase domain (TKD) or internal tandem duplication (ITD) mutation with either a high ratio (>0.7) or a low ratio (0.05 to 0.7) of mutant to wild-type alleles (ITD [high] and ITD [low], respectively). Allogeneic transplantation was allowed. The primary end point was overall survival.
Results
A total of 717 patients underwent randomization; 360 were assigned to the midostaurin group, and 357 to the placebo group. The FLT3 subtype was ITD (high) in 214 patients, ITD (low) in 341 patients, and TKD in 162 patients. The treatment groups were well balanced with respect to age, race, FLT3 subtype, cytogenetic risk, and blood counts but not with respect to sex (51.7% in the midostaurin group vs. 59.4% in the placebo group were women, P=0.04). Overall survival was significantly longer in the midostaurin group than in the placebo group (hazard ratio for death, 0.78; one-sided P=0.009), as was event-free survival (hazard ratio for event or death, 0.78; one-sided P=0.002). In both the primary analysis and an analysis in which data for patients who underwent transplantation were censored, the benefit of midostaurin was consistent across all FLT3 subtypes. The rate of severe adverse events was similar in the two groups.
Conclusions
The addition of the multitargeted kinase inhibitor midostaurin to standard chemotherapy significantly prolonged overall and event-free survival among patients with AML and a FLT3 mutation. (Funded by the National Cancer Institute and Novartis; ClinicalTrials.gov number, NCT00651261.)

《壹篇》系主要面向医务人员的公益性头条号,不以营利为目的,不进行任何有偿咨询和服务,不出售任何产品,与ASCO、CSCO等所有专业学会和机构没有任何关系和联系,也不代表任何官方学会发声。
文章图片均来自网络,不做商业用途,若有版权争议请与《壹篇》联系。
坚持点赞、赞赏和转发是一种态度和支持。